HIGHLIGHTS
-
Completes first two drill holes of its 2025 Angilak Exploration Program comprising ~10,000m of diamond drilling.
-
KU Discovery Target:
-
The maiden drill hole, KU-DD-001 successfully targeted stacked gravity and structural anomalies (Figure 2), intersecting shallow high-grade uranium mineralization as well as numerous zones of lower-grade mineralization throughout the hole. The hole intersected total composite mineralization of 7.1 m, including a continuous shallow zone (between 84.95 m and 87.45 m) of 0.7 m of high-grade mineralization that had max radioactivity up to 18,490 CPS (Figures 3 & 4).
-
The initial drilling at the KU Discovery Target confirms the Company’s thesis that the 31 km long RIB-Nine Iron corridor is prospective for hosting uranium mineralization within the Angikuni Basin.
-
The KU Discovery target is located within the Angikuni Basin, approximately 3 km from the northern margin, where historical trench sampling returned grades up to 30.7% U 3 O 8 .
-
In May 2025, ATHA completed a ground gravity and electromagnetic (EM) survey, designed to vector in on priority targets along the highly prospective 31 km Rib-Nine Iron Trend, under cover of the Angikuni Basin – a direct analog to the Athabasca Basin.
-
At KU, the survey identified a large – 2 km long by 500 m wide geophysical gravity anomaly. Additionally, a geological structural study was completed in May by SRK Consulting, highlighting numerous interpreted NW-SE faults and cross cutting E-W faults. The gravity survey demonstrates the identified anomalies are coincident with the modeled faults, and the historical high-grade surficial mineralization.
-
-
Lac 50 Deposit:
-
The first hole targeted the J4/Ray zones located along the Lac 50 Trend (Figure 2). The hole successfully extended mineralization down-dip by ~100 m, demonstrating that the Lac 50 Deposit remains open and unconstrained, both along strike and at depth.
-
J4R-DD-0091 intersected 3.8 m of composite mineralization including 0.4 m of >10,000 CPS up to a max of 14,826 CPS (Figure 5).
-
The 2025 Angilak Exploration Program will continue to target expansion of the mineralized envelop at the Lac 50 Deposit while also testing regional targets along the RIB-Nine Iron Corridor.
-
Troy Boisjoli, CEO commented: “ The results from our first two diamond drill holes completed as part of the 2025 Angilak Exploration Program highlight two things. First, the robust metal endowment at the Angilak Uranium Project, and second, the successful systematic exploration approach of our world class technical team. The discovery of a new zone of mineralization on a maiden drill hole in a new area and the continued expansion at the Lac 50 Deposit is truly remarkable. Both confirm the Company’s exploration thesis, substantially derisking the numerous prospective regional targets, providing a roadmap for executing on successful discoveries. All this at a time when the macro thesis for uranium as the fuel for the New Nuclear Renaissance is top of mind for the world. ATHA Energy and our Angilak Project is well positioned to be a critical player in this cycle.”
Cliff Revering, VP Exploration added: “ Since acquiring the Angilak Project in April 2024, we have focused on developing our understanding of the regional geology, structural architecture and mineralization controls within the Lac 50 Deposit area and Angikuni Basin. As a result, our first hole in the KU target area not only intersected a significant mineralized structural corridor, but also a large graphitic fault breccia zone displaying substantial vertical displacement of the basin unconformity – geological conditions which are extremely favorable for deposition of unconformity-related uranium mineralization. Our thesis of the Angikuni Basin being an emerging uranium district and highly prospective for discovery of additional uranium deposits continues to unfold. “
VANCOUVER, BRITISH COLUMBIA / ACCESS Newswire / June 24, 2025 / ATHA Energy Corp. (TSX.V:SASK)(FRA:X5U)(OTCQB:SASKF) (“ATHA” or the “Company“), is pleased to announce results for the first two diamond drill holes completed as part its 2025 Angilak diamond drill exploration program at its 100%-owned Angilak Uranium Project, Nunavut.
The maiden drill hole at the KU Discovery Target intersected five zones of uranium mineralization, including a shallow high-grade lense. The results demonstrate the Company’s thesis that the Angikuni Basin is a direct analog to the Athabasca Basin, and that the 31 km RIB-Nine Iron Trend remains prospective for additional discoveries. At the Lac 50 Deposit, the first hole drilled at the J4/Ray Zone along the Lac 50 Trend intersected four zones of uranium mineralization, expanding the down-dip extents of the envelop of mineralization. J4R-DD-091 demonstrates that the Lac 50 Deposit remains open and unconstrained.

Figure 1: Angilak Project Area – 2025 Exploration Target Area (Black Rectangles) & Mapped Historic
*Notes:
I Previous operators of the Angilak Project completed 24 diamond drill holes in the Dipole Showing and intersected grades of up to 5.53% U3O8 over 0.5 m3
II Along the western margin, historic drilling at the RIB Discovery intersected shallow (<25 m depth>3O81, hosted within graphitic pelitic rocks with Athabasca style alteration
III Mushroom Lake surface outcrop spans an area of 3 km on surface with historical outcrops samples grading up to 47.8% U3O84 & 6
IV Nine-Iron showing with 5 historical diamond drill holes. Intersections of shallow uranium mineralization, grades up to 1.25% U3O8 and historical outcrops with grades up to 30.3% U3O83

Figure 2: Angilak Project Area – 2025 Drill Collar Locations
Table 1: 2025 Angilak Exploration Program Drill Collar Information
|
Hole ID |
Trend |
Zone |
Azimuth (°) |
Dip (°) |
Easting (mE) |
Northing (mN) |
Elevation (m) |
Final Depth (m) |
|
KU-DD-001 |
RIB-Nine Iron |
KU Target |
30 |
70 |
515830 |
6936190 |
256.5 |
599 |
|
J4R-DD-091 |
Lac 50 |
J4/Ray |
25 |
57 |
522295 |
6938558 |
218.0 |
650 |
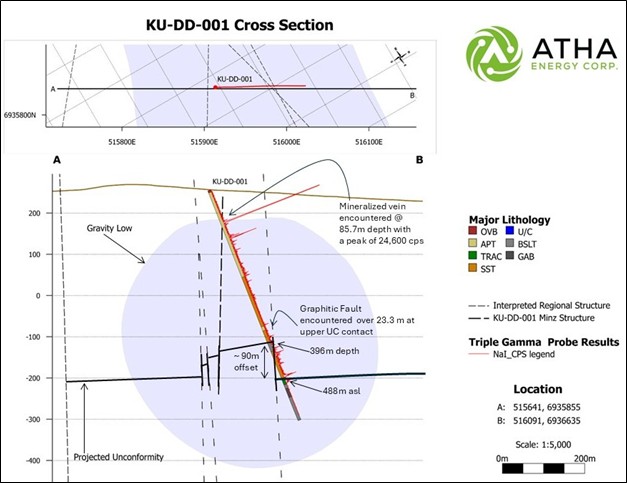
Figure 3: KU-DD-001 Cross-section displaying interpreted structure and downhole drill results
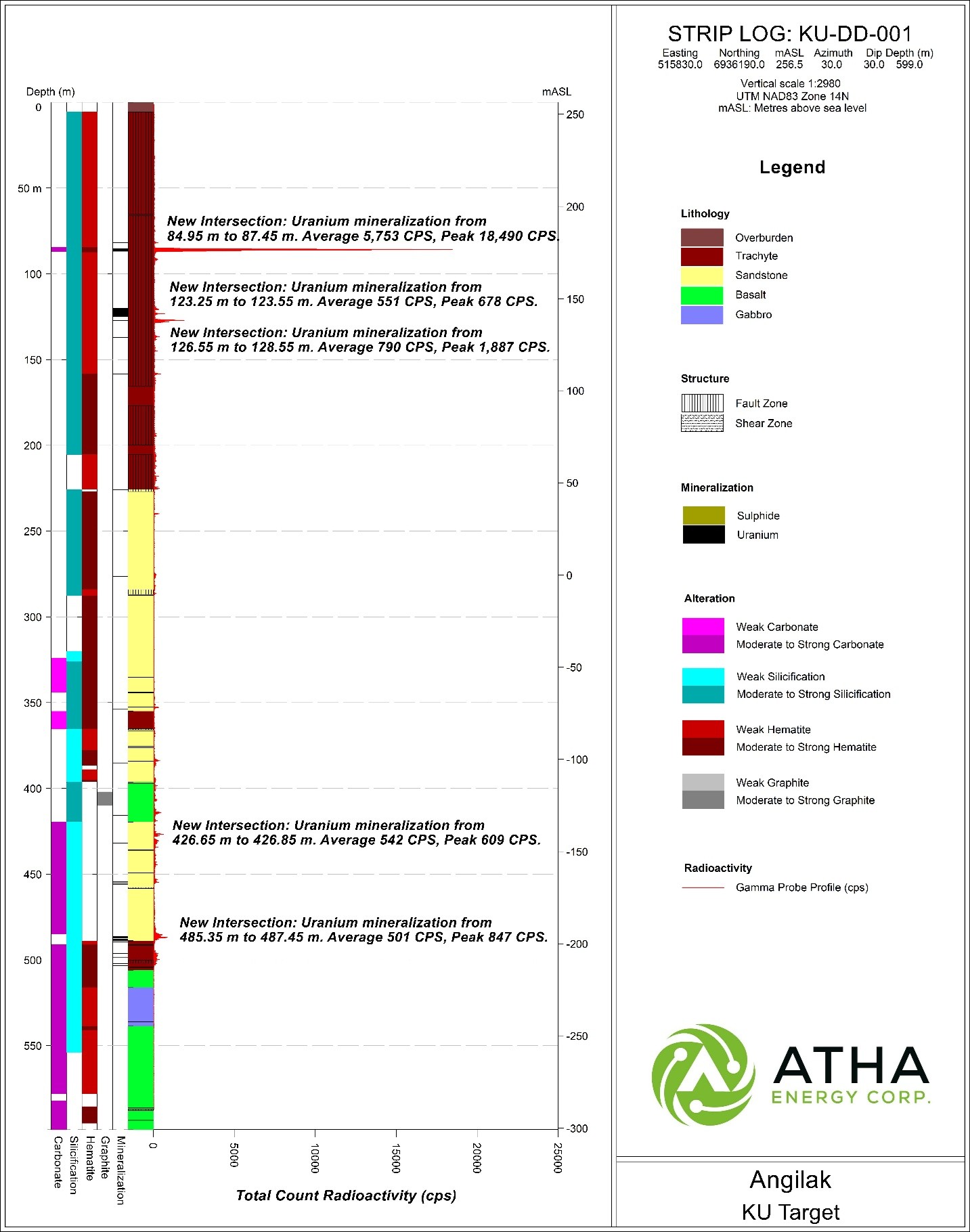
Figure 4: Striplog KU-DD-001
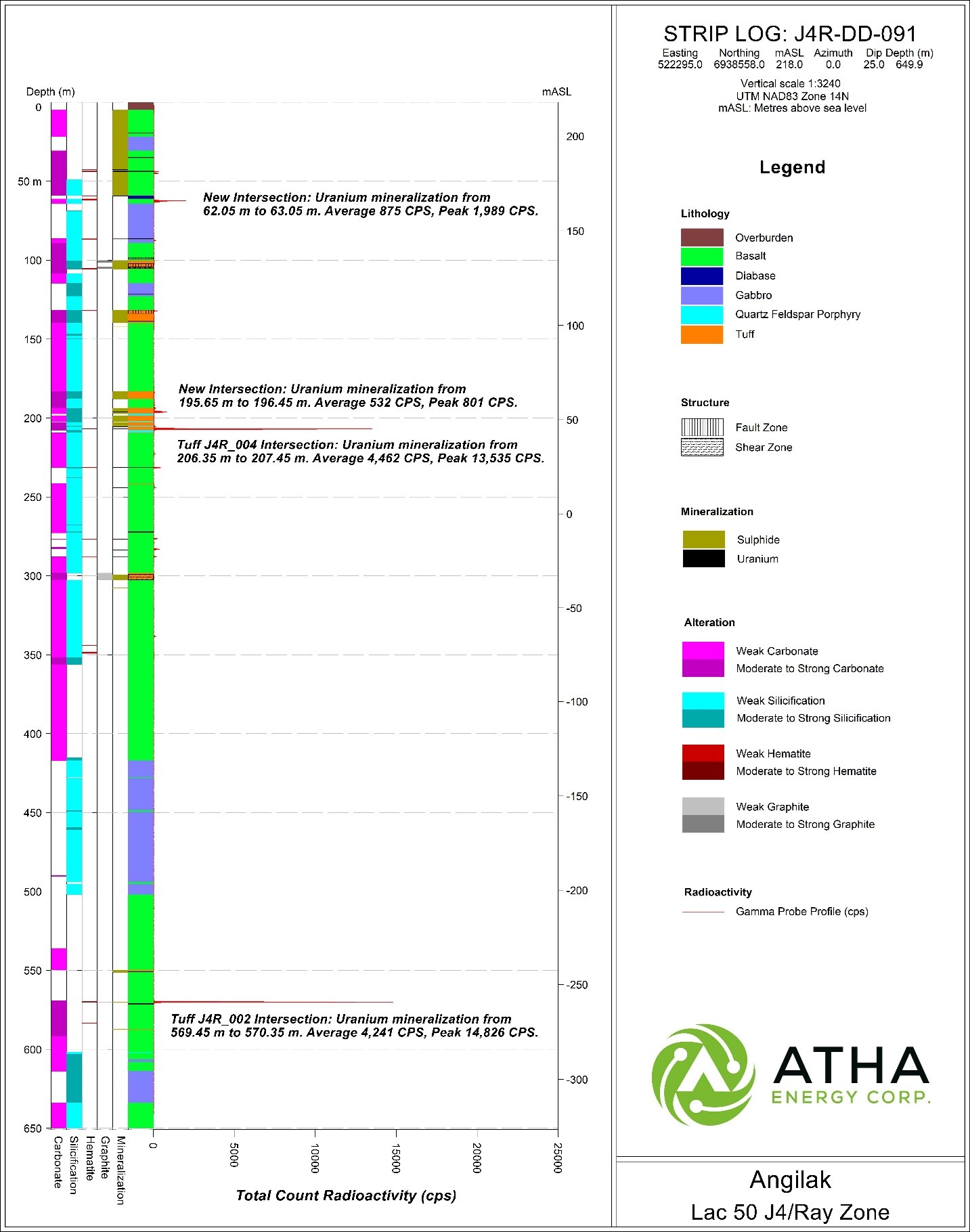
Figure 5: Striplog J4R-DD-091
Disclaimer for Historical Drilling and Outcrop Samples
Certain noted technical information provided herein has been derived exclusively and without independent verification from the following reports. Such information is historical in nature and is not considered by the Company to be current. In each case, the reliability of the historical information is considered reasonable by the Company. The historical information provides an indication of the exploration potential of the properties but may not be representative of expected results. Readers should read the entirety of such noted reports to fully understand the nature of the information referenced herein. Samples, including, without limitation, outcrop samples, by their nature, are selective in nature and significant variations may be seen from sample to sample. Accordingly, sample information may not be representative of the true underlying mineralization.
References for Historic Diamond Drilling Results
1. Papish, N.Z. 1978. 1978 Diamond Drill Report, Keewatin District N.W.T. Yathkyed Lake Area. Noranda Exploration company Assessment Report. March 6, 1979. A copy of such report is available on the website of the Government of Nunavut at https://nunavutgeoscience.ca/en/.
2. Dufresne, M.B., Sim, R. and Davis B., (2013). Technical report And Resource Update for the Angilak Project, Kivalliq Region, Nunavut. Technical Report prepared on behalf of Kivalliq Energy Corporation, March 1st, 2013. A copy of such report is available on the SEDAR+ profile of Kivalliq Energy Corporation at www.sedarplus.com.
3. Dufresne, M.B. and Schoeman, P. (2023). Technical report on the Angilak Project, Kivalliq Region, Nunavut. Technical Report prepared on behalf of ATHA Energy Corp. and Labrador Uranium Inc., January 31st, 2024. A copy of such report is available on the SEDAR+ profile of the Company at www.sedarplus.com.
References for Historic Surficial Sampling
4. Ward, j., Maynes, A., McNie, E., Forbes, A. and Stacey, J. 2012. Report on 2010 and 2011 Exploration Activity on Kivalliq Corporation’s Angilak IOCG-Uranium Property, Keewatin District, Nunavut. Kivalliq Energy Corporation Assessment Report. A copy of such report is available on the website of the Government of Nunavut at https://nunavutgeoscience.ca/en/.
5. Dufresne, M.B., Sim, R. and Davis B., (2013). Technical report And Resource Update for the Angilak Project, Kivalliq Region, Nunavut. Technical Report prepared on behalf of Kivalliq Energy Corporation, March 1st, 2013. Copy of such report is available on the SEDAR+ profile of Kivalliq Energy Corporation at www.sedarplus.com
6. Dufresne, M.B. and Schoeman, P. (2023). Technical report on the Angilak Project, Kivalliq Region, Nunavut. Technical Report prepared on behalf of ATHA Energy Corp. and Labrador Uranium Inc., January 31, 2024. A copy of such report is available on the SEDAR+ profile of the Company at www.sedarplus.com.
Qualified Person
The scientific and technical information contained in this news release have been reviewed and approved by Cliff Revering, P.Eng., Vice President, Exploration of ATHA, who is a “qualified person” as defined under National Instrument 43-101 – Standards of Disclosure for Mineral Projects.
About ATHA
ATHA is a Canadian mineral company engaged in the acquisition, exploration, and development of uranium assets in the pursuit of a clean energy future. With a strategically balanced portfolio including three 100%-owned post discovery uranium projects (the Angilak Project located in Nunavut, and CMB Discoveries in Labrador, and the newly discovered basement hosted GMZ high-grade uranium discovery located in the Athabasca Basin). In addition, the Company holds the largest cumulative prospective exploration land package (>7 million acres) in two of the world’s most prominent basins for uranium discoveries – ATHA is well positioned to drive value. ATHA also holds a 10% carried interest in key Athabasca Basin exploration projects operated by NexGen Energy Ltd. and IsoEnergy Ltd. For more information visit www.athaenergy.com.
On Behalf of the Board of Directors
Troy Boisjoli, CEO, ATHA Energy Corp
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
For more information, please contact:
Troy Boisjoli
Chief Executive Officer
Email: info@athaenergy.com
www.athaenergy.com
1-236-521-0526
Cautionary Statement Regarding Forward-Looking Information
This press release contains “forward-looking information” within the meaning of applicable Canadian securities legislation. Generally, forward-looking information can be identified by the use of forward-looking terminology such as “plans”, “expects” or “does not expect”, “is expected”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates” or “does not anticipate”, or “believes”, or variations of such words and phrases or state that certain actions, events or results “may”, “could”, “would”, “might” or “will be taken”, “occur” or “be achieved”. These forward-looking statements or information may relate to ATHA’s proposed exploration program, including statements with respect to the expected benefits of ATHA’s proposed exploration program, any results that may be derived from ATHA’s proposed exploration program, the timing, scope, nature, breadth and other information related to ATHA’s proposed exploration program, any results that may be derived from the diversification of ATHA’s portfolio, the prospects of ATHA’s projects, including mineral resources estimates and mineralization of each project, the prospects of ATHA’s business plans and any expectations with respect to defining mineral resources or mineral reserves on any of ATHA’s projects, and any expectation with respect to any permitting, development or other work that may be required to bring any of the projects into development or production.
Forward-looking statements are necessarily based upon a number of assumptions that, while considered reasonable by management at the time, are inherently subject to business, market and economic risks, uncertainties and contingencies that may cause actual results, performance or achievements to be materially different from those expressed or implied by forward-looking statements. Such assumptions include, but are not limited to, assumptions that the anticipated benefits of ATHA’s proposed exploration program will be realized, that no additional permit or licenses will be required in connection with ATHA’s exploration programs, the ability of ATHA to complete its exploration activities as currently expected and on the current anticipated timelines, including ATHA’s proposed exploration program, that ATHA will be able to execute on its current plans, that ATHA’s proposed explorations will yield results as expected, and that general business and economic conditions will not change in a material adverse manner. Although ATHA has attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking information, there may be other factors that cause results not to be as anticipated, estimated or intended. There can be no assurance that such information will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliance on forward-looking information.
Such statements represent the current view of ATHA with respect to future events and are necessarily based upon a number of assumptions and estimates that, while considered reasonable by ATHA, are inherently subject to significant business, economic, competitive, political and social risks, contingencies and uncertainties. Risks and uncertainties include, but are not limited to the following: inability of ATHA to realize the benefits anticipated from the exploration and drilling targets described herein or elsewhere; in ability of ATHA to complete current exploration plans as presently anticipated or at all; inability for ATHA to economically realize on the benefits, if any, derived from the exploration program; failure to complete business plans as it currently anticipated; overdiversification of ATHA’s portfolio; failure to realize on benefits, if any, of a diversified portfolio; unanticipated changes in market price for ATHA shares; changes to ATHA’s current and future business and exploration plans and the strategic alternatives available thereto; growth prospects and outlook of the business of ATHA; and the ability to advance the Company projects and its proposed exploration program; risks inherent in mineral exploration including risks related worker safety, weather and other natural occurrences, accidents, availability of personnel and equipment, and other factors; aboriginal title; failure to obtain regulatory and permitting approvals; no known mineral resources/reserves; reliance on key management and other personnel; competition; changes in laws and regulations; uninsurable risks; delays in governmental and other approvals, community relations; stock market conditions generally; demand, supply and pricing for uranium; and general economic and political conditions in Canada, Australia and other jurisdictions where ATHA conducts business. Other factors which could materially affect such forward-looking information are described in the filings of ATHA with the Canadian securities regulators which are available on ATHA’s profile on SEDAR+ at www.sedarplus.ca. ATHA does not undertake to update any forward-looking information, except in accordance with applicable securities laws.
SOURCE: ATHA Energy Corp
View the original press release on ACCESS Newswire